Framework for Rational Donor Selection in Fecal Microbiota Transplant Clinical Trials
By Katie E. Golden
Fecal Microbiota Transplant (FMT) is gaining traction as a potentially effect treatment of the increasing number of diseases that have been linked to microbiome dysbiosis. FMT has demonstrated consistently high cure rates in the treatment of recurrent C.difficile infection, and is already under investigation for use in Inflammatory Bowel Disease as well. Given FMT’s potential to expand its clinical application to a wide variety of conditions, researchers are recognizing the unique opportunities and challenges in designing clinical trials to study its efficacy. In a recently published article1, scientists outline a conceptual framework for designing future trials to optimize success when investigating this novel therapy.
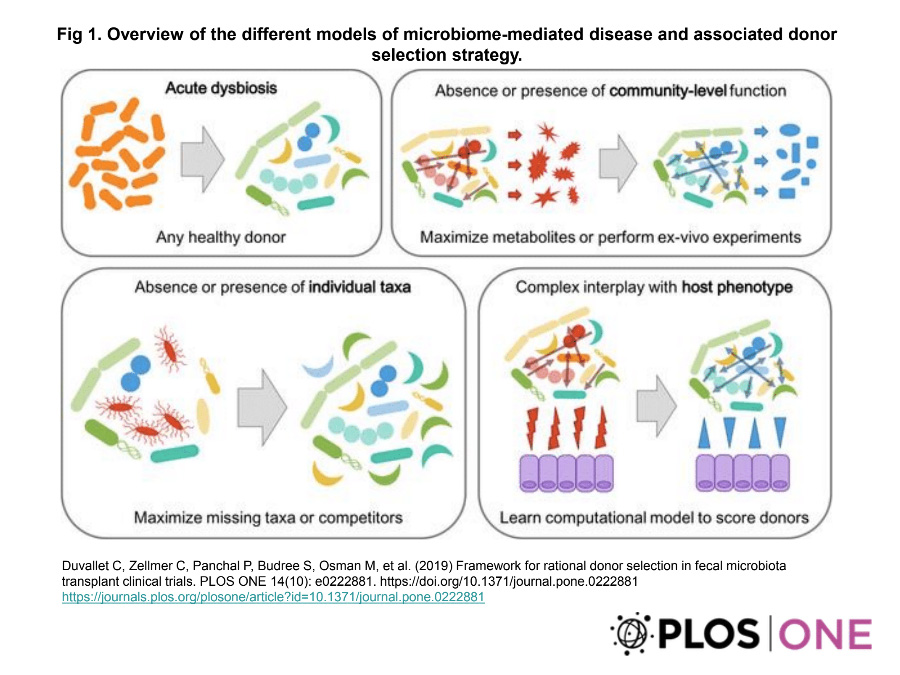
There are inherent obstacles to studying an intervention like FMT. As it is performed by transferring whole stool samples from a healthy donor to the patient, there are a number of factors and potential mechanisms through which it may be effective: colonization of donor bacterial strains, non-bacterial molecular components, or even unique clinical features of the donor. Given the large variation in microbial composition among healthy donor samples, as well as the heterogeneity in targeted diseases and their corresponding microbiome dysregulation, choosing the most appropriate donor samples is paramount to effectively understanding the potential efficacy of FMT. Here, the authors describe several different strategies for donor selection based on the hypothesized disease model.
For diseases defined by broad dysbiosis, as is the case in C.difficile infection, there is unstable overgrowth of pathogenic communities and loss of protective mechanisms typically achieved by commensal species. In this model, choosing any healthy donor should be effective to induce some degree of homeostasis that corresponds to clinical improvement. Other diseases have been linked to the specific absence of harmful special, or presence of beneficial species. Research has shown, for example, protective benefits of specific taxa in cholera infections, as well as a causal role of a bacteria for inducing colorectal cancer progression. In these models, donor samples should be selected to either remove or replace these specific taxa. This requires screening samples for an abundance of beneficial bacteria, a minimal level of culprit bacteria, or choosing samples with bacteria known to out-compete the harmful populations.
Not all microbiome-associated disease is defined by overgrowth, absence, or presence of a specific bacterial species. Instead, they may be characterized by a community level dysfunction. Hepatic encephalopathy, for example, is a common complication of liver cirrhosis and has been tied to deficient microbiome’s production of short-chain fatty acids and bile acids. In these conditions, it would likely be more beneficial to screen stool samples for the desired function, rather than for specific bacterial taxa. Finally, in more complicated disease models with a dynamic interplay between host and microbiome populations, a more nuanced approach will likely be required. The authors describe the use of computational models to predict microbiome characteristics of specific host phenotypes.
By developing a broad framework for designing future FMT trials, the authors effectively advocate for thoughtful and informed methodology to accelerate our understanding of this promising treatment.
1. Duvallet C, Zellmer C, Panchal P, Budree S, Osman M, Alm EJ. Frame for rational donor selection in fecal microbiota transplant clinical trials. PLoS ONE 14(10): e0222881.

