Development of a Covalent Inhibitor of Gut Bacterial Bile Salt Hydrolases
By Dr. Katie E Golden, MD
Advancing microbiome research has identified the critical role of molecular messengers responsible for the interaction between intestinal bacteria and host immunity. Secondary bile acids, in particular, have emerged as key players that allow resident microbes to modulate host physiology. Understanding the metabolic activity of bile acids has been limited by a lack of investigatory tools that target their synthesis and function. In a recent study published in Nature Chemical Biology, CMIT faculty and colleagues detail the novel development of an enzyme inhibitor that can be used to overcome this current road block, allowing us to investigate the role of bile acids in microbiome-related disease.1
Primary bile acids are produced in the liver, stored in the gallbladder, and released into the small intestine to aid in digestion. The majority of these molecules are reabsorbed in the small intestine and transported back to the liver. A portion of primary bile acids, however, pass into the large intestine where they are modified by our gut bacteria. The bacteria produce an enzyme called bile salt hydrolase (BSH) that conjugates the primary bile acids to secondary bile acids, which are metabolically active and have been implicated in multiple different disease states. These molecules bind to several different types of host receptors, and can augment physiologic pathways from glucose and lipid metabolism to immune response and inflammatory cascades.
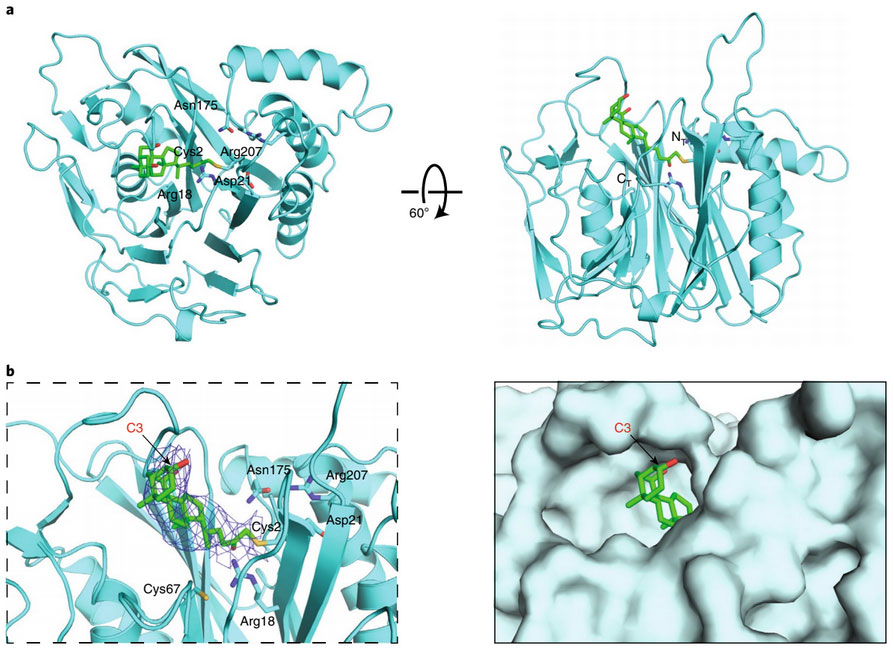
To better understand exactly how these secondary bile acids affect host physiology, the researchers of this study sought to engineer a non-toxic, ubiquitous inhibitor of bacterial BSH. They focused specifically on the design of a covalent inhibitor, which provides the unique advantage of high selectivity for its protein target, as well as high potency in the presence of ample native substrate. They targeted a catalytic cysteine residue on the BSH active site, which is a highly conserved across colonic bacterial strains. After they designed nine different potential inhibitors, they analyzed each candidate’s activity and mechanism across a variety of gram-positive and gram-negative bacterial strains. After several rounds of investigation, they successfully found the inhibitor that was highly effective at reducing bile acid conjugation in mouse models. The researchers were able to demonstrate, furthermore, that this inhibitor was not systemically absorbed, did not induce unintended modulation of off-target host receptors, and did not restrict bacterial growth.
Their research yields a useful tool that can be used to better understand the role of these secondary bile acids at the microbiome-host interface, and their implications for host health and disease. Prior studies, for example, have shown how mice colonized with BSH-knockout strains gain less weight and have a favorable energy metabolism profile, and a more recent study has even suggested that secondary bile acids may inhibit protective anti-tumor activity in the liver. These findings would suggest that the scope of the physiologic impact of these metabolites is largely undiscovered, and that BSH inhibition shows exciting potential as a potential therapeutic target in a variety of human disease.
1. Adhikari, A.A., Seegar, T.C.M., Ficarro, S.B. et al. Development of a covalent inhibitor of gut bacterial bile salt hydrolases. Nat Chem Biol 16, 318–326 (2020).