Engineering Phage Host-Range and Suppressing Bacterial Resistance through Phage Tail Fiber Mutagenesis
By Dr. Katie E. Golden, MD
The rapid increase in antibiotic resistance poses an urgent pressure on the scientific community to develop alternative therapies for the increasing frequency of life-threatening bacterial infections. Researchers have been increasingly interested in the use of bacteriophages to provide a solution. Phages are viruses that target and kill bacteria without being pathogenic to the host organism. While they are promisingly unaffected by traditional antibiotic-resistant mechanisms, they are not immune to their own pitfalls and resistance. Phages are selective for particular bacterial strains, and recognize their target by cell surface receptors, which can ultimately mutate to evade infection. In a recently published paper by Yehl et.al.1 in Cell, investigators outline a novel strategy that addresses this engineering roadblock.
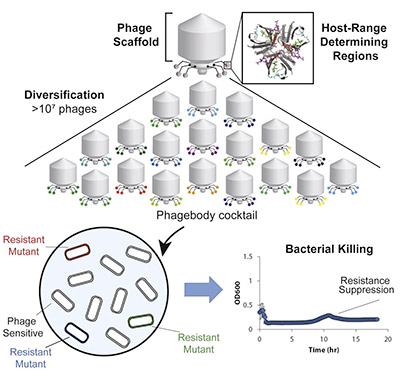
Before now, phage-based therapies were developed to be combinations of different phages with varied binding to reduce the selective pressure on the bacterial target. This has proven ineffective given our limited knowledge of the biology and pharmacodynamics of the uncharacterized phages. Researchers are subsequently looking for technology that maintains targeted specificity while also broadening phage host-range. In this paper, the authors describe how they performed targeted mutagenesis of specific phage regions that are critical for bacterial recognition, creating diversity at binding regions that slows evolution of bacterial resistance mechanisms.
They focused on a specific region of the phage tail fiber, where there are variable regions that can be mutated to alter recognition specificity. By engineering this region with mutagenesis, they sought to expand the host-range of a single type of bacteriophage to target phage-resistant bacterial strains, and prevent the evolution of further resistance. Their results are promising. In subsequent experiments, the engineered phages enabled long-term suppression of bacterial growth, as well as decreased resistance development. They furthermore demonstrated the phages’ functionality in mouse models. This exciting approach has opened the door for the advancement of novel antimicrobial treatments to address the escalating concern for untreatable bacterial infection.

