Cryo-electron Microscopy Structures of Human Oligosaccharyltransferase Complexes OST-A and OST-B
By Dr.Katie E. Golden, MD
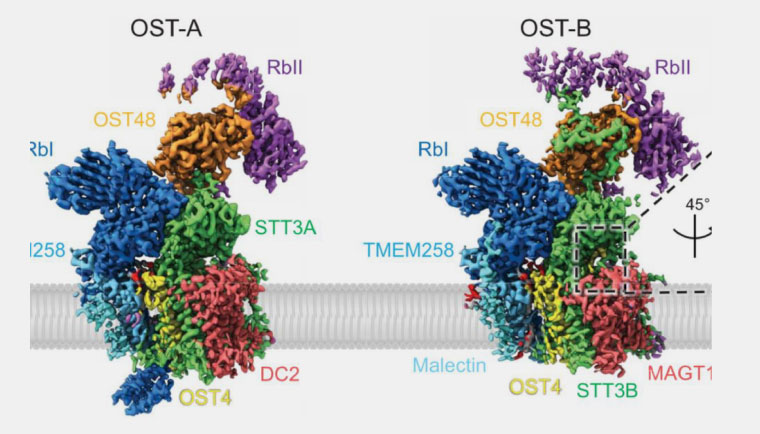
Back in 2016, CMIT researchers proposed a mechanism by which a gene-dense locus on chromosome 11, TMEM258, plays an important role in the pathogenesis of Inflammatory Bowel Disease.1 They illustrated how it is a component of the oligosaccharyltransferase (OST) complex that is required for protein glycosylation, and without it, mice models demonstrated severe colonic inflammation.
In a more recent paper published in Science , researchers use cryo-electron microscopy to define the chemical structure of two human OST complexes that play an important role in secretory protein modification.2 This is not only an exciting discovery that builds on Graham et.al. ’s work from 2016, but is also an important example of advanced basic science research that unmasks physiologic mechanisms with potential clinical relevance.
To break it down, when proteins are translated from genetic material in the cell, those proteins need to be modified before they are functional. A process called N-glycosylation is one of the most common ways that proteins are modified, and is catalyzed by OST complexes located in the endoplasmic reticulum (a specialized membrane found inside mammalian cells that aids in protein synthesis). Humans have two distinct OST complexes: OST-A and OST-B. Despite similar molecular structures, these complexes have distinct functions in the glycosylation process. In this most recent paper by Ramírez et.al., the authors discovered the specific catalytic subunits (STT3A and STT3B) that account for the unique substrate affinities of each complex. OST-A associates with the translocon and binds unfolded proteins to process the N-glycosylation sites needed for protein modification. OST-B, contrastingly, does not associate with the translocon but has a stronger affinity for glycosylation sites and is thus able to bind and process proteins that are partially folded and modified.
While the above is an oversimplification of a complicated process, this paper decisively identifies the subtle but specific structural changes that translate to unique enzymatic function. Given the ubiquitous nature of these cellular processes, Ramírez and his team have laid important groundwork for the design of N-glycosylation inhibitors, which could ultimately be used to augment specific disease processes in the clinical setting.
- Graham DB, Lefkovith A, Deelen P, de Klein N, Varma M, Boroughs A, Desch AN, Ng ACY, Guzman G, Schenone M, Petersen CP, Bhan AK, Rivas MA, Daly MJ, Carr SA, Wijmenga C, Xavier RJ. TMEM258 Is a Component of the Oligosaccharyltransferase Complex Controlling ER Stress and Intestinal Inflammation. Cell Rep. 2016 Dec 13;17(11):2955-2965.
- Ramírez AS, Kowal J, Locher KP. Cryo-electron microscopy structures of human oligosaccharyltransferase complexes OST-A and OST-B. Science. 2019 Dec 13;366(6471):1372-1375.

